Answer:
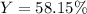
Step-by-step explanation:
Hello,
For the given chemical reaction:
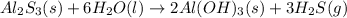
We first must identify the limiting reactant by computing the reacting moles of Al2S3:
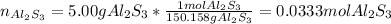
Next, we compute the moles of Al2S3 that are consumed by 2.50 of H2O via the 1:6 mole ratio between them:
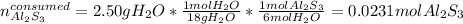
Thus, we notice that there are more available Al2S3 than consumed, for that reason it is in excess and water is the limiting, therefore, we can compute the theoretical yield of Al(OH)3 via the 2:1 molar ratio between it and Al2S3 with the limiting amount:
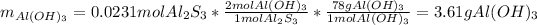
Finally, we compute the percent yield with the obtained 2.10 g:
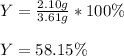
Best regards.