Answer: c. At equilibrium, the concentration of reactants is greater than the products
Step-by-step explanation:
Equilibrium constant for a reaction is the ratio of concentration of products to the concentration of reactants each raised to the power its stoichiometric coefficients.
For the reaction:
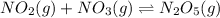
Equilibrium constant is given as:
![K_(eq)=([N_2O_5])/([NO_2]* [NO_3])](https://img.qammunity.org/2021/formulas/chemistry/college/rb70e7qp8bxxxip32kmehnyfyfe72zlbju.png)
![2.1* 10^(-20)=([N_2O_5])/([NO_2]* [NO_3])](https://img.qammunity.org/2021/formulas/chemistry/college/cb6ib89iju8astt32aefjg3mmdur1guq6o.png)
When
a) K > 1, the concentration of products is greater than the concentration of reactants
b) K < 1, the concentration of reactants is greater than the concentration of products
c) K= 1, the reaction is at equilibrium, the concentration of reactants is equal to the concentration of products
Thus as
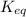 is
is
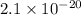 which is less than 1,
which is less than 1,
the concentration of reactants is greater than the concentration of products