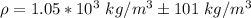Complete Question
The complete question is shown on the first uploaded image
Answer:
The experimental value of density is
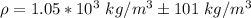
Comparing it with the value of density of water (
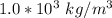 ) we can see that the density of ice is greater
) we can see that the density of ice is greater
Step-by-step explanation:
From the question we are told
The mass is
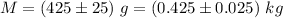
The volume is
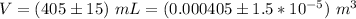
The experimental value of density is mathematically evaluated as
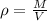
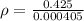
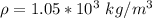
The possible error in this experimental value of density is mathematically evaluated as
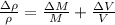
substituting value
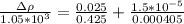
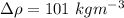
Thus the experimental value of density is