Answer:
The boiling and freezing temperatures of krypton at absolute scale are 119.95 K and 116.05 K, respectively.
Step-by-step explanation:
The absolute temperature on SI units corresponds to Kelvin scale, whose conversion formula in terms of the Celsius scale is:
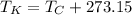
Where:
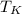 - Absolute temperature, measured in Kelvins.
- Absolute temperature, measured in Kelvins.
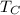 - Relative temperature, measured in Celsius.
- Relative temperature, measured in Celsius.
Finally, freezing and boiling temperatures are converted into absolute scale:
Boiling temperature
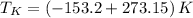
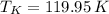
Freezing temperature
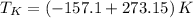
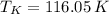
The boiling and freezing temperatures of krypton at absolute scale are 119.95 K and 116.05 K, respectively.