Answer:
Radius at liftoff 8.98 m
Step-by-step explanation:
At the working altitude;
maximum radius = 24 m
air pressure = 0.030 atm
air temperature = 200 K
At liftoff;
temperature = 349 K
pressure = 1 atm
radius = ?
First, we assume balloon is spherical in nature,
and that the working gas obeys the gas laws.
from the radius, we can find the volume of the balloon at working atmosphere.
Volume of a sphere =
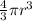
volume of balloon =
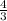 x 3.142 x
x 3.142 x
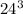 = 57913.34 m^3
= 57913.34 m^3
using the gas equation,
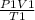 =
=
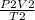
The subscript 1 indicates the properties of the gas at working altitude, and the subscript 2 indicates properties of the gas at liftoff.
imputing values, we have
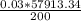 =
=
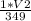
0.03 x 57913.34 x 349 = 200V2
V2 = 606352.67/200 = 3031.76 m^3 this is the volume occupied by the gas in the balloon at liftoff.
from the formula volume of a sphere,
V =
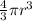 =
=
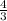 x 3.142 x
x 3.142 x
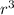 = 3031.76
= 3031.76
4.19
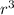 = 3031.76
= 3031.76
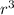 = 3031.76/4.19
= 3031.76/4.19
radius r of the balloon on liftoff =
![\sqrt[3]{723.57}](https://img.qammunity.org/2021/formulas/physics/college/hw50g6csjkysy49t7dibywb1us3c6va6bh.png) = 8.98 m
= 8.98 m