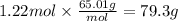Answer:
NaN₃(s) ⇒ Na(s) + 1.5 N₂(g)
79.3 g
Step-by-step explanation:
There is some info missing. I think this is the original question.
The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas.
1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide (NaN₃) into solid sodium and gaseous dinitrogen.
2. Suppose 43.0L of dinitrogen gas are produced by this reaction, at a temperature of 13.0°C and pressure of exactly 1atm. Calculate the mass of sodium azide that must have reacted. Round your answer to 3 significant digits.
Step 1: Write the balanced equation
NaN₃(s) ⇒ Na(s) + 1.5 N₂(g)
Step 2: Convert the temperature to Kelvin
We will use the following expression.
K = °C + 273.15
K = 13.0°C + 273.15
K = 286.2 K
Step 3: Calculate the moles of N₂ produced
We will use the ideal gas equation.
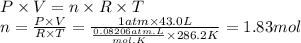
Step 4: Calculate the moles of NaN₃ required to produce 1.83 moles of N₂
The molar ratio of NaN₃ to N₂ is 1:1.5. The moles of NaN₃ required are 1/1.5 × 1.83 mol = 1.22 mol
Step 5: Calculate the mass corresponding to 1.22 moles of NaN₃
The molar mass of NaN₃ is 65.01 g/mol.