Answer:
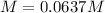
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
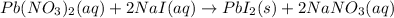
Thus, for 0.904 g of precipitate, that is lead (II) iodide, we can compute the initial moles of lead (II) ions in lead (II) nitrate:
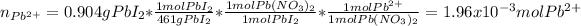
Finally, the resulting molarity in 30.8 mL (0.0308 L):
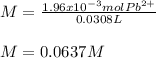
Regards.