Answer:
The energy of the light is not higher than the work function. Then, the electrons are not emitted.
Step-by-step explanation:
In order to calculate the maximum kinetic energy of the electrons for photons of twice the wavelength of the light, you first calculate the wavelength of photons with energy of 6eV. You use the following formula:
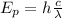 (1)
(1)
c: speed of light = 3*10^8 m/s
λ: wavelength of the light
h: Planck's constant in eV.s = 4.135*10^-15 eV.s
E: energy of the photons = 6eV
You solve the equation (1) for λ:
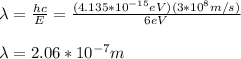
Next, you calculate the energy of photons with twice the wavelength:
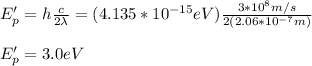
Next, you calculate the work function of the metal by using the equation for the photo electric effect:
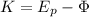 (2)
(2)
Ф: work function
Ep: energy of the photons = 6eV
K: kinetic energy of emitted electrons = 2eV
You solve for Ф:
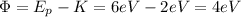 (3)
(3)
Finally, you calculate the kinetic energy of the emitted electron by the metal when the light with energy Ep' is used:
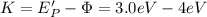
It is clear that for a light with energy 3.0eV has an energy lower than the work function of the metal, then, the electrons are not emmited by the metal