Answer:
0.15M
Step-by-step explanation:
Molar Concentration =

Assuming 20g of the unadulterated aluminium chloride was weighed into the volumetric flask, and given molar mass of AlCl3 = 133.34g/mol
∴ Molarity =
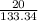
= 0.14999 ≈ 0.15M (to 2 significant figures)
I hope this solution is clear. The same can be calculated from concentration by volume.