Answer:
1.85 J/K
Step-by-step explanation:
The computation of total change in entropy is shown below:-
Change in Entropy = Sum Q ÷ T
=
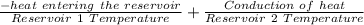

= -3.12 + 4.97
= 1.85 J/K
Therefore for computing the total change in entropy we simply applied the above formula.
As we can see that there is heat entering the reservoir so it will be negative while cold reservoir will be positive else the process would be impossible.