Answer:
The concentration of the base will be lower than the exact one.
Step-by-step explanation:
Hello,
In this case, in neutralization processes, the following equation is used:

It means that the moles of acid equal the moles of base at the equivalence point and in terms of volumes and concentrations is:
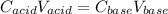
In such a way, by decreasing the volume of acid, the computed or estimated concentration of the base will be lower than the original value. for that reason, accurate procedures must be performed.
Regards.