Answer:
See figure 1
Step-by-step explanation:
In this question, we have to find first the chiral carbons on 1,2-difluoro-1,2-dimethylcyclopentane. Carbons 1 and 2. Then, using the Cahn–Ingold–Prelog priority rules. We can draw all the stereoisomers. If we have 2 chiral carbons we will have (
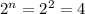 ) 4 isomers. With this in mind we can have the R,R isomer; the S,S isomer; the S,R isomer, and the R,S isomer. We have to remember that:
) 4 isomers. With this in mind we can have the R,R isomer; the S,S isomer; the S,R isomer, and the R,S isomer. We have to remember that:
Priority 1 = "F"
Priority 2= "C"
Priority 3= "CH2"
Priority 4 = "CH3"
I hope it helps!