Answer:
K = 13448.64eV
Step-by-step explanation:
(a) In order to calculate the kinetic energy of the electrons, to "see" the atom, you take into account that the wavelength of the electrons must be of the order of the resolution required (0.010nm).
Then, you first calculate, by using the Broglies' relation, the momentum of the electron associated to a wavelength of 0.010nm:
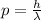 (1)
(1)
p: momentum of the electron
h: Planck's constant = 6.626*10^-34 Js
λ: wavelength = 0.010nm
You replace the values of the parameters in the equation (1):
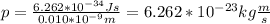
With this values of the momentum of the electron you can calculate the kinetic energy of the electron by using the following formula:
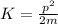 (2)
(2)
m: mass of the electron = 9.1*10^-31 kg
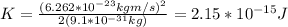
In electron volts you obtain:
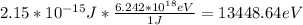
The kinetic energy required for the electrons must be, at least, of 13448.64 eV