Answer:
a.
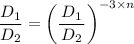 which is constant therefore, n = constant
which is constant therefore, n = constant
b. The temperature at the end of the process is 109.6°C
c. The work done by the balloon boundaries = 10.81 MJ
The work done on the surrounding atmospheric air = 10.6 MJ
Step-by-step explanation:
p₁ = 100 kPa
T₁ = 27°C
D₁ = 10 m
v₂ = 1.2 × v₁
p ∝ α·D
α = Constant
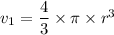
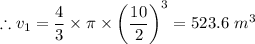
v₂ = 1.2 × v₁ = 1.2 × 523.6 = 628.32 m³
Therefore, D₂ = 10.63 m
We check the following relation for a polytropic process;
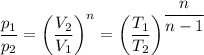
We have;
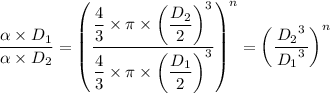
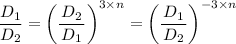
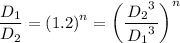
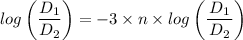
n = -1/3
Therefore, the relation, pVⁿ = Constant
b. The temperature T₂ is found as follows;
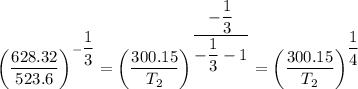
T₂ = 300.15/0.784 = 382.75 K = 109.6°C
c.
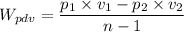
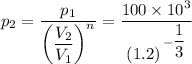
p₂ = 100000/0.941 = 106.265 kPa
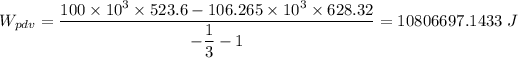
The work done by the balloon boundaries = 10.81 MJ
Work done against atmospheric pressure, Pₐ, is given by the relation;
Pₐ × (V₂ - V₁) = 1.01×10⁵×(628.32 - 523.6) = 10576695.3 J
The work done on the surrounding atmospheric air = 10.6 MJ