Answer:
15 moles.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
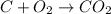
Clearly, since carbon and oxygen are in a 1:1 molar ratio, 15 moles of carbon will completely react with 15 moles of oxygen, therefore 15 moles of oxygen remain as leftovers. In such a way, since carbon and carbon dioxide are also in a 1:1 molar ratio, the theoretical yield of carbon dioxide is 15 moles based on the stoichiometry:
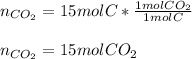
Best regards.