Answer:
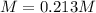
Step-by-step explanation:
Hello,
In this case, for each nitrate-based salt, we compute the nitrate moles as shown below:
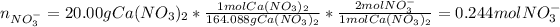
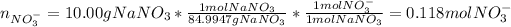
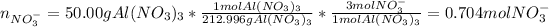
We notice calcium nitrate has two moles of nitrate ion, sodium nitrate has one and aluminium nitrate has three. Hence we add the moles to obtain the total moles nitrate ion:
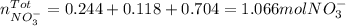
Finally, we compute the molarity:
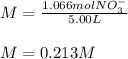
Regards.