Answer:
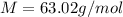
Step-by-step explanation:
Hello,
In this case, for the calculation of the molar mass of nitric acid, we should employ the following formula, knowing that there is one hydrogen atom, one nitrogen atom and three oxygen atoms:
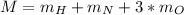
Now, we use the atomic mass of each atom to compute the total molar mass:
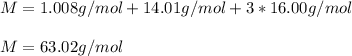
Best regards.