Answer:
To answer this question we assumed that the area units and the thickness units are given in inches.
The number of atoms of lead required is 1.73x10²³.
Step-by-step explanation:
To find the number of atoms of lead we need to find first the volume of the plate:
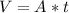
Where:
A: is the surface area = 160
t: is the thickness = 0.002
Assuming that the units given above are in inches we proceed to calculate the volume:
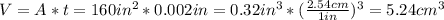
Now, using the density we can find the mass:
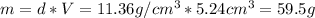
Finally, with the Avogadros number (
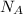 ) and with the atomic mass (A) we can find the number of atoms (N):
) and with the atomic mass (A) we can find the number of atoms (N):
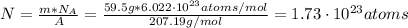
Hence, the number of atoms of lead required is 1.73x10²³.
I hope it helps you!