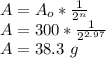Answer:
38.3 g
Step-by-step explanation:
The half life of a substance is the time taken for the substance to decay to about half of its amount.
Let us assume that I-125 have a half life of 60 days. It means that every 60 days, the amount of I - 125 would be halved.
The amount of I - 125 (A) that remains after t days is given by:
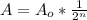
Where Ao is the initial amount and n = t / half life
Given that:
Ao = 300 g, t = 178.2 days.
Therefore n = t / half life = 178.2 days / 60 days = 2.97