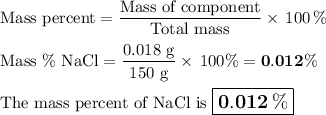Answer:
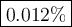
Step-by-step explanation:
Data:
Solution 1: V₁ = 50.0 mL; c₁ = 0.05 mol·L⁻¹
Solution 2: V₂ = 100 mL; c₂ = 0.02 mol·L⁻¹
NaCl : ρ = 2.1 g/mL
1. Solution 1
(a) Moles of NaCl
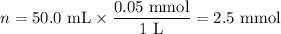
(b) Mass of NaCl
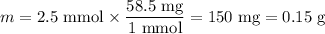
(c) Volume of NaCl
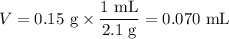
(d) Volume of water
V = 50.0 mL - 0.070 mL = 49.9 mL
(e) Mass of water
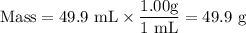
2. Solution 2
(a) Moles of NaCl
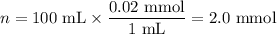
(b) Mass of NaCl
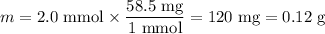
(c) Volume of NaCl
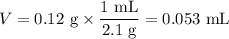
(d) Volume of water
V =100 mL - 0.055 mL = 100 mL
(e) Mass of water
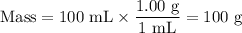
3. Combined solutions
(a) Mass of NaCl
Mass of NaCl = 0.015 g + 0.012 g = 0.018 g
(b) Mass of water
Mass of water = 49.9 g + 100 g = 150 g
(c) Mass percent