Answer:
There are
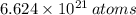 of Germanium in a germanium-silicon alloy that contains 15 wt% Ge and 85 wt% Si.
of Germanium in a germanium-silicon alloy that contains 15 wt% Ge and 85 wt% Si.
Step-by-step explanation:
The masses of silicon and germanium contained in a cubic centimeter of the germanium-silicon alloy by apply the concepts of mass (
 ), density (
), density (
 ) and volume (
) and volume (
 ), as well as the mass-mass proportion of Germanium (
), as well as the mass-mass proportion of Germanium (
 ):
):
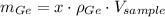
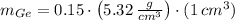
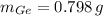
The amount of moles of Germanium is obtained after dividing previous outcome by its atomic weight. That is to say:
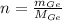
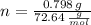
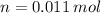
There are 0.011 moles in a cubic centimeter of the germanium-silicon alloy. According to the Law of Avogadro, there are
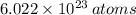 in a mole of Germanium. The quantity of atoms in a cubic centimeter is therefore found by simple rule of three:
in a mole of Germanium. The quantity of atoms in a cubic centimeter is therefore found by simple rule of three:
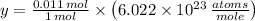
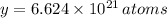
There are
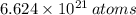 of Germanium in a germanium-silicon alloy that contains 15 wt% Ge and 85 wt% Si.
of Germanium in a germanium-silicon alloy that contains 15 wt% Ge and 85 wt% Si.