Answer:
The answer is 3.87 J/g°C
Step-by-step explanation:
Here is the equation we are going to use:
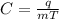
C= specfic heat in J/g°C
q = heat in joules (J)
m = mass in grams (g)
T = change in temperature
Here is what is given:
q = 849 J
m = 95.4 g
T = 48.0 - 25.0 = 23°C
Find:
Specific heat capacity in J/g
The first thing we are going to do is plug everything into the equation:
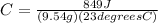
Then we are going to solve for C
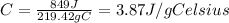
Hope this helps!