Answer:
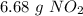
Step-by-step explanation:
We have to start with the combustion reaction:
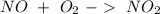
Then we can balance the reaction:
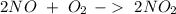
If we want to find the theoretical yield, we have to calculate the amount of
 . To do this, we have to first convert the 4.36 g of
. To do this, we have to first convert the 4.36 g of
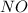 to moles
to moles
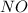 (using the molar mass 30 g/mol), then we have to convert from moles of
(using the molar mass 30 g/mol), then we have to convert from moles of
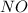 to moles of
to moles of
 (using the molar ratio) finally, we have to convert from moles of
(using the molar ratio) finally, we have to convert from moles of
 to grams of
to grams of
 (using the molas mass 46 g/mol), so:
(using the molas mass 46 g/mol), so:
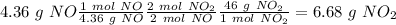
I hope it helps!