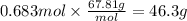Answer:
0.683 mol
46.3 g
Step-by-step explanation:
There is some info missing. I think this is the original question.
Boron trifluoride gas is collected at 21.0 °C in an evacuated flask with a measured volume of 50.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.330 atm. Calculate the mass and number of moles of boron trifluoride gas that were collected. Round your answer to 3 significant digits.
Step 1: Convert the temperature to the Kelvin scale
We will use the following expression.
K = °C + 273.15
K = 21.0°C + 273.15
K = 294.2 K
Step 2: Calculate the moles of boron trifluoride gas
We will use the ideal gas equation.
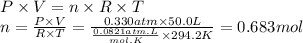
Step 3: Calculate the mass of boron trifluoride gas
The molar mass of BF₃ is 67.81 g/mol.