Answer:
3) 310 g/mol
Step-by-step explanation:
Hello,
In this case, for calcium carbonate, we are able to compute its gram-formula mass by considering the atomic mass of each element composing it and their subscripts as shown below:
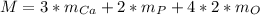
Thus, we compute:
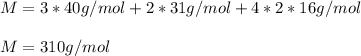
Hence answer is 3) 310 g/mol . Remember this is also known as the molar mass of the mentioned compound.
Best regards.