Answer:
Step-by-step explanation:
a. Determine the number of valence electrons for each atom in the molecule
In this case we both atoms are halogens. Therefore we will have 7 electrons for each atom.
b. Draw the Lewis Dot structure
In this case, the formula is
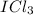 , so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1
, so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1
c. Describe why the molecule is drawn this way (i.e. any extra rules/steps needed?)
In this specific case, the "I" atom don't follow the octet rule. We will have an expanded octet for iodine (more than 8 electrons).
d. Show the polarity of each bond and for the molecule by drawing in the dipole +d
The negative dipole would be placed in the atom with higher electronegativity, in this case "Cl". The positive dipole would be placed in the atom with low electronegativity, in this case "I".
I hope it helps!