Given that,
Sun exposure = 30%, 45%, 60%, 75%, 90%
Stem mass (g) = 275, 415, 563, 815, 954
Stem volume (ml) = 1100, 1215, 1425, 1610, 1742
(a). We need to convert the mass measurements to kilograms (kg) and the volume measurements to cubic meters
Using conversion of mass
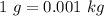
Conservation of volume
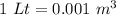
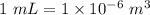
So, mass in kg
Stem mass (kg) = 0.275, 0.415, 0.563, 0.815, 0.954
Volume in m³,
Stem volume (m³) = 0.0011, 0.001215, 0.001425, 0.001610, 0.001742
(b). We need to calculate the density of the samples
Using formula of density
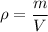
Where, m = mass
V = volume
If the m = 0.275 kg and V = 0.0011 m³
Put the value into the formula
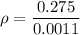
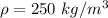
If the m = 0.415 kg and V = 0.001215 m³
Put the value into the formula
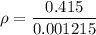
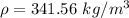
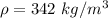
If the m = 0.563 kg and V = 0.001425 m³
Put the value into the formula
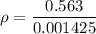
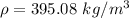
If the m = 0.815 kg and V = 0.001610 m³
Put the value into the formula
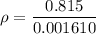
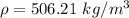
If the m = 0.954 kg and V = 0.001742 m³
Put the value into the formula
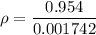
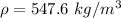
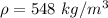
(c). We need to convert the density values to scientific notation
In scientific notation
The densities are
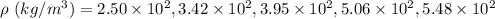
Hence, This is required solution.