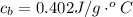Complete Question
The complete question is shown on the first uploaded image
Answer:
The specific heat is
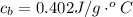
Step-by-step explanation:
From the question we are told that
The mass of the sample is
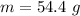
The mass of the water is
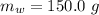
The initial temperature of the sample is

The initial temperature of the water is
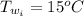
The final temperature of the water is
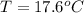
Note the final temperature of water is equal to the final temperature of brass sample
The pressure is
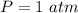
Generally for according to the law of energy conservation
The heat lost by sample = The heat gain by water
The heat lost by brass sample is mathematically evaluated as
![H_L = m * c_b * [T_i - T]](https://img.qammunity.org/2021/formulas/chemistry/college/qx9aocvvieyff1cij4sbt8w0axs45o82jy.png)
Where
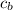 is the specific neat of the brass sample
is the specific neat of the brass sample
The heat gained by water is mathematically evaluated as
![H_g = m_w *c_w * [T_w - T ]](https://img.qammunity.org/2021/formulas/chemistry/college/3vfvjxc74f1zsqg9y2d7cfmm1ayyivndr6.png)
where
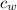 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of
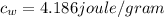
So
![H_L = H_g \ \equiv m* c_b * [T_i -T] = m_w * c_w * [T - T_w]](https://img.qammunity.org/2021/formulas/chemistry/college/a4xi3t8j0ba7vcvnnf1vkavdm3gbray5xv.png)
substituting values
![52.4 * c_b * [95.1 - 17.6] = 150 * 4.186 * [ 17.6 - 15.0]](https://img.qammunity.org/2021/formulas/chemistry/college/t953vd9pq67r2o52o19tv83eccpexz1npt.png)