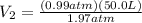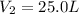Answer: 25.0 L
Step-by-step explanation:
For this problem, we will need to use Boyle's Law.
Boyle's Law: P₁V₁=P₂V₂
Since we are looking for V₂, we can rewrite the equation.
V₂=P₁V₁/P₂
The pressure should be in atm, so let's convert kPa to atm.
P₁=100 kPa=0.99 atm
P₂=200 kPa=1.97 atm
Now that we know the pressure in atm, we can plug it into the equation and solve.