Answer:
The lighter a gas is, the faster it will tend to diffuse.
Using either the Molar mass below or the diffusion rates, you will see that CH4 is the lightest and with greatest diffusion rate hence the fastest.
THE ANSWER IS CH4
Step-by-step explanation:
Use Grahams Law to determine diffusion rates
Diffusion rate =
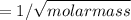
Molar mass:
CH4 = 16.04 g/mol
C2H2 = 26.04 g/mol
NO = 30.01 g/mol
O2 = 32 g/mol
Diffusion Rates using formula above:
CH4 = 0.24968
C2H2 = 0.19596
NO = 0.18254
O2 = 0.17677