Answer:
1. 3.3 cm³; 2. 3.5 g/cm³; 3. barium; 4. 4%
Step-by-step explanation:
Experimental data:
Mass = 11.3 g
Length = 13.90 cm
Width = 2.9 cm
Thickness = 0.081 cm
Calculations:
1. Volume of bar
V = lwh = 13.90 cm × 2.9 cm × 0.081 cm = 3.3 cm³
2. Experimental density
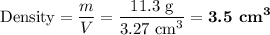
3. Identity of metal
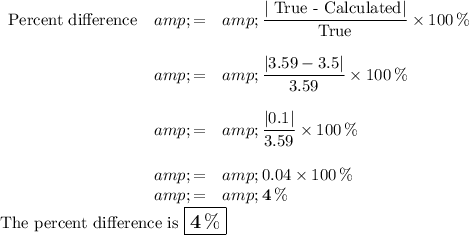
The three most likely metals are scandium (3.00 g/cm³), barium (3.59 g/cm³), and yttrium (4.47 g/cm³)
The metal is probably barium.
4. Percent difference