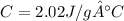Answer: 2.02 J/g°C
Step-by-step explanation:
To find the heat capacity, we have to manipulate the equation for heat.
q=mCΔT becomes C=q/(mΔT) to find heat capacity. Since we are given our values, we can plug in to find C.
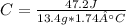
*Please ignore the capital A in front of the °C. In order to have ° in the equaiton, the A pops up.