Given that,
Mass of water = 1.26 kg
Latent heat of vaporization of water
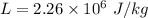
We need to calculate the latent heat
Using formula of latent heat
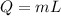
Where, Q = energy
m = mass
L = latent heat of vaporization
Put the value into the formula
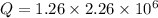
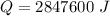
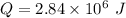
The temperature changes liquid to steam.
So, The temperature will be increases.
Hence, The latent heat is
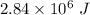 and temperature increases.
and temperature increases.