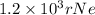Answer:
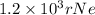
Step-by-step explanation:
Given that
Speed of neon = 350 m/s
Un-certainity in speed= (0.01 ÷ 100) × 350
= 0.035 m/s
As per heisenberg uncertainty principle
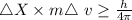 ....... (i)
....... (i)
substituting the values in equation (i)
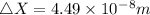
In terms of rNe i.e 38 pm =
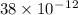
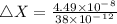
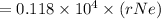
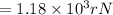
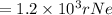
Therefore the smallest possible length of the box inside in which the atom could be known for locating with certainty is