Answer:
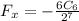
Attractive
Step-by-step explanation:
Data provided in the question
The potential energy of a pair of hydrogen atoms given by
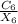
Based on the given information, the force that one atom exerts on the other is
Potential energy μ =
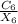
Force exerted by one atom upon another
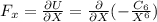
or
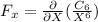
or
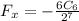
As we can see that the
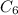 comes in positive and constant which represents that the force is negative that means the force is attractive in nature
comes in positive and constant which represents that the force is negative that means the force is attractive in nature