Answer:
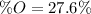
Step-by-step explanation:
Hello,
In this case, for the sample of the given compound, we can compute the moles of each atom (carbon, hydrogen and oxygen) that is present in the sample as shown below:
- Moles of carbon are contained in the 9.582 grams of carbon dioxide:
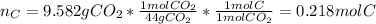
- Moles of hydrogen are contained in the 3.922 grams of water:
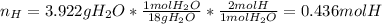
- Mass of oxygen is computed by subtracting both the mass of carbon and hydrogen in carbon dioxide and water respectively from the initial sample:
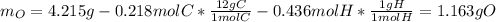
Finally, we compute the percent by mass of oxygen:
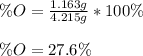
Regards.