Answer:
A) 2.41 * 10^-2 M
Step-by-step explanation:
Here we go from grams of percipitate to moles of Silver nitrate because we already know NaCl is excess so we don't care about that.
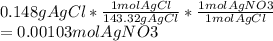
Now that we have moles we know molarity is moles/litres so we just plug it in:
Molarity = mol/litres = 0.00103/0.0428 L = 0.0241 M or 2.41 * 10^-2 M