Answer:
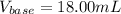
Step-by-step explanation:
Hello,
In this case, since you have both the final and initial volume for the titration procedure, clearly, the used volume is computed by the subtraction between them, since it accounts for the employed volume of the base, it turns out:
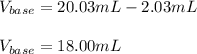
Best regards.