Answer:
The net work done is 272.38 kJ
Step-by-step explanation:
The parameters given are;
Mass of water = 5 kg
p₁ = 20 bar
T₁ = 360°C
v₁ = 0.141147 m³/kg
Process 1 to 2 = Constant pressure process
p₂ = 20 bar
Process 2 to 3 = Constant volume process
p₃ = 5 bar
Process 3 to 4 = Constant pressure process
Process 4 to 1 = Polytropic process pv = Constant
For Stage 1 to 2, we have;
p₂ = 20 bar
From the steam tables for superheated steam, we have;
T₂ = 212.385°C
v₂ = 0.0995805 m³/kg
Work done = p₂×(v₂ - v₁) = 2×10⁶ × (0.0995805 - 0.141147 ) = -83133 J/kg
For the 5 kg, we have;
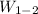 = -83133 J/kg × 5 = -415,665 J
= -83133 J/kg × 5 = -415,665 J
Stage 2 to 3: Constant volume cooling
v₂ = v₃ = 0.0995805 m³/kg
p₃ = 5 bar
T₃ = 151.836°C
(0.0995805 - 0.00109256)/(0.374804 - 0.00109256) = 0.2635 liquid vapor mixture
Work done,
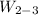 = 0
= 0
Stage 3 to 4: Constant pressure heating
p₃ = p₄ = 5 bar
v₄/T₄ = v₃/T₃
v₄ = 0.374804 m³/kg
T₄ = v₄×T₃/v₃ = 0.374804*(273.15 + 151.836)/0.0995805 = 1599.6 K = 1326.4 °C
Work done = p₄×(v₄ - v₃) = 5×10⁵ × (0.374804 - 0.0995805 ) = 137611.75 J/kg
For the 5 kg, we have;
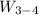 = 137,611.75 J/kg × 5 = 688,058.75 J
= 137,611.75 J/kg × 5 = 688,058.75 J
Stage 4 to 1: Polytropic process
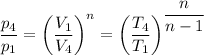
Which gives;
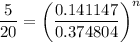
n = log(5/20) ÷log(0.141147/0.374804) = 1.42
Work done,
 , is given as follows;
, is given as follows;
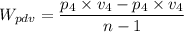
Which gives;
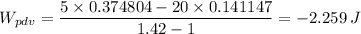
For the 5 kg, we have;
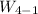 = -2.259 J/kg × 5 = -11.2967 J
= -2.259 J/kg × 5 = -11.2967 J
The net work done,
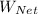 , is therefore;
, is therefore;
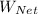 =
=
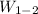 +
+
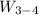 +
+
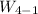
-415,665 + 688,058.75 -11.2967 = 272,382.45 J = 272.38 kJ.