Answer:
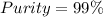
Step-by-step explanation:
Hello,
In this case, the undergoing precipitation reaction is:
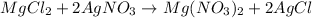
Thus, for the 0.2500 moles of silver nitrate, the following mass of magnesium chloride is consumed (consider their 2:1 molar ratio):
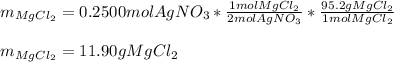
Therefore, the purity of the sample is:
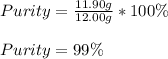
Best regards.