Answer:
Depending on the
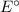 value of
value of
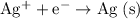 , the cell potential would be:
, the cell potential would be:
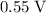 , using data from this particular question; or
, using data from this particular question; or- approximately
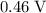 , using data from the CRC handbooks.
, using data from the CRC handbooks.
Step-by-step explanation:
In this galvanic cell, the following two reactions are going on:
- The conversion between
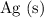 and
and
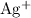 ions,
ions,
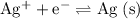 , and
, and - The conversion between
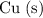 and
and
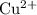 ions,
ions,
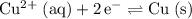 .
.
Note that the standard reduction potential of
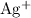 ions to
ions to
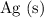 is higher than that of
is higher than that of
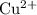 ions to
ions to
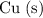 . Alternatively, consider the fact that in the metal activity series, copper is more reactive than silver. Either way, the reaction is this cell will be spontaneous (and will generate a positive EMF) only if
. Alternatively, consider the fact that in the metal activity series, copper is more reactive than silver. Either way, the reaction is this cell will be spontaneous (and will generate a positive EMF) only if
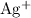 ions are reduced while
ions are reduced while
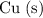 is oxidized.
is oxidized.
Therefore:
- The reduction reaction at the cathode will be:
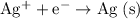 . The standard cell potential of this reaction (according to this question) is
. The standard cell potential of this reaction (according to this question) is
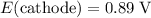 . According to the 2012 CRC handbook, that value will be approximately
. According to the 2012 CRC handbook, that value will be approximately
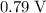 .
.
- The oxidation at the anode will be:
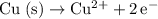 . According to this question, this reaction in the opposite direction (
. According to this question, this reaction in the opposite direction (
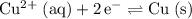 ) has an electrode potential of
) has an electrode potential of
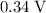 . When that reaction is inverted, the electrode potential will also be inverted. Therefore,
. When that reaction is inverted, the electrode potential will also be inverted. Therefore,
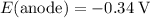 .
.
The cell potential is the sum of the electrode potentials at the cathode and at the anode:
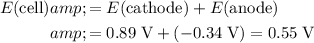 .
.
Using data from the 1985 and 2012 CRC Handbook:
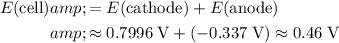 .
.