Answer:
-
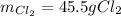
-
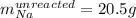
Step-by-step explanation:
Hello,
In this case, we consider the undergoing chemical reaction as:
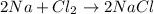
Thus, for 75.0 grams of sodium chloride, the following grams of chlorine are required (consider their 2:1 molar ratio):
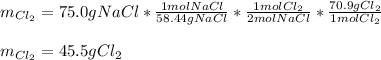
Then, for the unreacted grams of sodium, we first compute the actually reacted grams by considering the 2:2 molar ratio between sodium chloride and sodium:
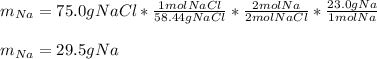
Finally, we subtract:
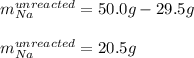
Regards.