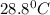Answer: Thus the final temperature for both the mercury and the water is
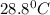
Step-by-step explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
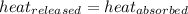
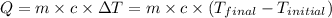
![-[m_1* c_1* (T_(final)-T_1)]=[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2021/formulas/chemistry/college/r415gvr8ppfjm6b6z5sw2bh44ko5buh8v7.png)
Q = heat absorbed or released
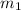 = mass of mercury= 452 g
= mass of mercury= 452 g
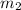 = mass of water = 145 g
= mass of water = 145 g
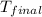 = final temperature = ?
= final temperature = ?
 = temperature of mercury =
= temperature of mercury =
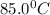
 = temperature of water =
= temperature of water =
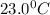
 = specific heat of mercury =
= specific heat of mercury =
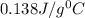
 = specific heat of water=
= specific heat of water=
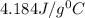
Now put all the given values in equation (1), we get
![-[452* 0.138* (T_f-85.0)^0C]=145* 4.184* (T_f-23.0)^0C](https://img.qammunity.org/2021/formulas/chemistry/college/qttobos80m1dyaaz5r3l1a4pqaw0xxqh8z.png)
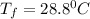
Thus the final temperature for both the mercury and the water is