Answer:
a) First-order.
b) 0.013 min⁻¹
c) 53.3 min.
d) 0.0142M
Step-by-step explanation:
Hello,
In this case, on the attached document, we can notice the corresponding plot for each possible order of reaction. Thus, we should remember that in zeroth-order we plot the concentration of the reactant (SO2Cl2 ) versus the time, in first-order the natural logarithm of the concentration of the reactant (SO2Cl2 ) versus the time and in second-order reactions the inverse of the concentration of the reactant (SO2Cl2 ) versus the time.
a) In such a way, we realize the best fit is exhibited by the first-order model which shows a straight line (R=1) which has a slope of -0.0013 and an intercept of -2.3025 (natural logarithm of 0.1 which corresponds to the initial concentration). Therefore, the reaction has a first-order kinetics.
b) Since the slope is -0.0013 (take two random values), the rate constant is 0.013 min⁻¹:
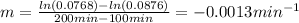
c) Half life for first-order kinetics is computed by:
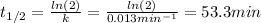
d) Here, we compute the concentration via the integrated rate law once 1500 minutes have passed:
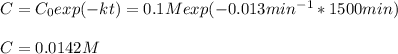
Best regards.