Answer:
The amount of Mg was enough
Step-by-step explanation:
In this case, we have to start with the reaction between
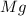 and
and
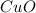 , so:
, so:
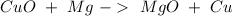
If we check the reaction is already balanced. Now, we can do some stoichiometry to calculate the amount of Mg. The first step is the number of moles of
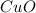 . To this we have to calculate the molar mass of
. To this we have to calculate the molar mass of
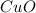 first, so:
first, so:
Cu: 63.55 g/mol and O: 16 g/mol. So, (63.55+16)= 79.55 g/mol.
Now, we can calculate the moles:
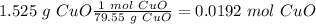
The molar ratio between
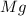 and
and
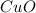 is 1:1, so:
is 1:1, so:
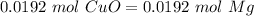 .
.
Now we can calculate the mass of Mg if we know the atomic mass of Mg (24.305 g/mol). So:
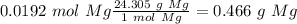
With this in mind, the student added enough Mg to recover all the Cu.
Note: The HCl doesn't take a role in the reaction. The function of HCl is to dissolve the
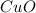 .
.
I hope it helps!