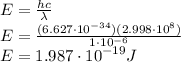Answer:
Energy is found as:
E = 1.987·10⁻¹⁹ J
Step-by-step explanation:
Energy of a single photon of infrared light can be found by using the following formula:
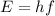
where
E = in Joules
h = Planck's constant = 6.627×10 ⁻³⁴ J
f = frequency in hertz
It can also be written as:
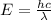
where
c = 2.998×10⁸ ms⁻¹
λ = wavelength
Wavelength is given in the question which is:
λ = 1×10⁻⁶m
Substitute all the values in the Energy formula