Answer:
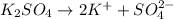
concentration of
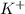 = 0.50 M
= 0.50 M
concentration of
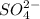 = 0.25 M
= 0.25 M
Step-by-step explanation:
The dissociation equation of
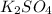 is:
is:
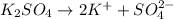
According to stoichiometry:
1 mole of
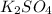 gives 2 moles of
gives 2 moles of
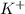
Thus 0.25 moles of
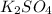 gives =
gives =
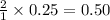 moles of
moles of
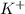
Similarly,
1 mole of
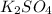 gives = 1 mole of
gives = 1 mole of
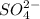
Thus 0.25 moles of
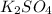 gives =
gives =
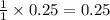 moles of
moles of
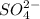
Thus the concentration of
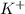 and
and
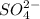 are 0.50 M and 0.25 M respectively.
are 0.50 M and 0.25 M respectively.