Answer:
See exaplanation
Step-by-step explanation:
For this question, we several sub-questions, lets start with the first one:
What is its molecular formula?
For this question we have to multiply the subunit by the number of subunits in the polysaccharide, so:
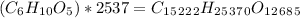
What is its molar mass?
For this question, we have to find the molar mass of 1 subunit and then multiply by the number of subunits:
Atomic masses: C: 12 g/mol H: 1 g/mol O: 16 g/mol
Now we can multiply, the atomic masses by the number of atoms, so:
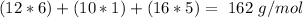
If we take into account the number of subunits:
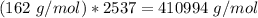
What is the empirical formula of amylose?
In this case, we have to remember that the empirical formula is the smallest number of atoms. In other words, we have to simplify the formula. Therefore, the smallest formula is the subunit formula:
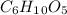 .
.
I hope it helps