Answer:
The final temperature = 93.45°C
The constant volume work done = -568.76 kJ/kg (work is done by the gas)
The constant pressure work done = -756.186 kJ/kg (work is done by the gas)
Step-by-step explanation:
The parameters given are;
Pressure = 2.0 MPa = 20 Bars
Temperature = 500 °C
Process = Reversible adiabatic (isentropic) process = Constant entropy, s
Therefore;
s₁ = s₂
From the steam tables for super heated steam, s₁ = 7.4335 kJ/(kg·K)
At T₂, we have saturated vapor, hence;
Where:
s₁ = s₂ = 7.4335 kJ/(kg·K), we have;
The final temperature, T₂, is given as follows;
T₂ = 93.4854 + (7.4339 -7.4335 )/(7.4790 - 7.4339) * (89.9315-93.4854) = 93.45°C
The work done, W in an isentropic process is given as follows
p·dV work,
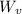 = m·
= m·
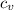 ·(T₂ - T₁)
·(T₂ - T₁)
V·dp work,
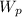 = m·
= m·
 ·(T₂ - T₁)
·(T₂ - T₁)
The specific heat at constant pressure,
 , for steam = 1.86 kJ/(kg·K)
, for steam = 1.86 kJ/(kg·K)
R = 0.461 kJ/(kg·K)
∴
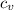 =
=
 - R = 1.86 - 0.461 = 1.399 kJ/(kg·K)
- R = 1.86 - 0.461 = 1.399 kJ/(kg·K)
Hence;
p·dV work = 1.399 * (93.45 - 500 ) = -568.76 kJ/kg
V·dp work = 1.86 * (93.45 - 500 ) = -756.186 kJ/kg
Also work done,
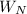 , can be expressed as follows;
, can be expressed as follows;
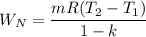
Where:
k =
 /
/
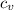 = 1.86/1.399 = 1.33
= 1.86/1.399 = 1.33
∴
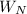 = 0.461 * (93.45 - 500 )/(1-1.33) = 567.94 kJ/kg done by the gas.
= 0.461 * (93.45 - 500 )/(1-1.33) = 567.94 kJ/kg done by the gas.