Answer:
Paraffin combustion produces more energy per gram than ethanol fuel but paraffin fuel has a larger carbon footprint
Step-by-step explanation:
Paraffin is also known by the standard chemical name alkane with a chemical formula for a paraffin given as follows;
CₙH₂ₙ₊₂
While gasoline, also known as petrol, consists of light weight alkanes and alkenes
The heat of combustion of gasoline is about 47.3 kJ/g
The combustion of gasoline is incomplete as presented in the following equation
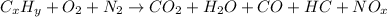
Example of complete combustion of octane, however, is given as follows;
2C₈H₁₈ + 25O₂ → 16CO₂ + 18H₂O + Energy
The source of paraffin is from petroleum
Ethanol fuel can be produced by the fermentation of Sugar
The heat of combustion of ethanol = 1366.91 kJ/mol = 29.664 kJ/g.
2CH₃OH + 3O₂ → 2CO₂ + 4H₂O + Energy